Farshad Roghani Dehkordia*, Amir Farhad Kamkhahb
aDepartment of Internal Medicine and Cardiology and Medicinal Plant Research Center, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran
bDepartment of Clinical Pathology, School of Medicine, Shahrekord University of Medical Sciences, Shahrekord, Iran
AB S T R A CT
Hypertension (HT) is a lifestyle-related disease and dietary modifications are effective for its management and prevention. We conducted a randomized, double-blind, placebo-controlled trial to evaluate the efficacy of treatment with an oral Nigella sativa (NS) seed extract supplement in patients with mild HT. Subjects were randomized into three groups: a placebo and two test groups that received 100 and 200 mg of NS extract twice a day. After 8 weeks, systolic blood pressure (SBP) values in both case groups were found to be significantly reduced when compared with the baseline values for each group. In addition, the decrease in SBP in the two case groups was statistically significant relative to the placebo group (P < 0.05–0.01). Meanwhile, diastolic blood pressure (DBP) values in the case groups were found to be significantly reduced from the baseline and a significant reduction was also observed in these groups (P < 0.01) when compared with the placebo group. In addition, extract administration reduced both SBP and DBP in a dose-dependent manner. Meanwhile, NS extract caused a significant decline in the level of total and low-density-lipoprotein (LDL)-cholesterol relative to baseline data. No complications caused by NS were observed. The results suggest that the daily use of NS seed extract for 2 months may have a blood pressure-lowering effect in patients with mild HT.
INTRODUCTION
Hypertension (HT) is a primary risk factor for heart disease, stroke, and renal failure, and is one of the most critical issues regarding human health [1,2]. HT is also a lifestyle-related disease and dietary modifications are effective for its management and prevention, i.e. engaging in moderate physical activity; maintaining normal body weight for adults; limiting alcohol consumption; reducing sodium intake; and consuming a diet rich in fruits, vegetables, and low-fat dairy products with a reduced content of saturated and total fat [2]. Patients with mild HT [systolic blood pressure (SBP)/diastolic blood pressure (DBP), 140–159/90–99 mmHg] with no risk factors and presence of risk factors other than diabetes are classified into low- and medium-risk groups, respectively. Lifestyle changes should be the first therapeutic measure in low- and medium-risk hypertensive patients. If SBP and DBP do not decline to levels lower than 140 and 90 mmHg, respectively, then antihypertensive drug treatments should be started [3].
As an alternative to drug therapy, it is desirable to change the dietary style and use medicinal plant products for the prevention and treatment of HT and thereby to avoid the adverse side-effects of organically synthesized drugs as well as the high cost of drug therapy [4–7]. In this regard, the use of plants as medicines dates back to the earliest years of human life. NS is one of the plants used as a medicinal herb for more than 2000 years, is considered safe, and has been shown to have multi-systemic beneficial actions [8] including hypoglycemic [9], hypocholesterolemic [10], and antioxidant [11] properties. There is some controversy about the cardiovascular actions of NS or its active ingredients, as some investigators reported no effect on blood pressure in animals [12] or humans [13], whereas others reported a dose-dependent decrease in the arterial blood pressure and heart rate in normal [14] or spontaneously hypertensive rats [15]. Therefore, NS may be considered as an antihypertensive medicinal plant. For this purpose, we conducted a single-center, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy of treatment with an oral NS seed extract supplement in patients with mild HT.
PATIENTS A N D M ETHODS
Study design: The current study was a randomized, double-blind, placebo-controlled, dose-response study, conducted for 8 weeks at the Chamran Hospital (Isfahan) in 2005. The study was conducted in compliance with the Declaration of Helsinki (1964) and its revised version (2002). The protocol was reviewed and approved by the Clinical Trial Ethics Committee of MUI University (Isfahan) before the start of the study. The nature of the study, the proposal of the study design, and the potential risks of participation in the study were fully explained to each volunteer and written consent was obtained.
Patients: Healthy male volunteers (n = 119), aged 35 to 50 years, with mild HT (SBP between 140 and 159 mmHg and/or DBP between 90 and 99 mmHg) [3] were included in this study. Subjects were excluded if they were heavy smokers (more than five cigarettes per day), if they had received antihypertensive and hypolipidemic therapy or food additives and vitamins, and if they exhibited abnormal liver function (aspartate aminotransferase >100 IU/L and/or alanine aminotransferase >100 IU/L), or abnormal renal function (blood urea nitrogen >22 mg/dL and/or serum creatinine >1.3 mg/dL).
Drug preparation: Fresh NS seeds were obtained from Tehran (northern Iran), washed, and dried at room temperature in the absence of sunlight. The plant was identified by botanists in the herbarium of Shaheed Beheshti University (Tehran) and the specimen number of the plant was 2005- Boiled extract of the seeds of the plant was prepared as follows: 10 g of the ground material was boiled with 100 mL of distilled water for 15 min and allowed to cool at room temperature. The extract was then filtered through a sterile cotton cloth until a concentrated residue (0.1 g%) was obtained. The standardized extract was then encapsulated in 100- and 200-mg packages and stored at 0C until being used. The capsules containing placebo were indistinguishable from those containing NS regarding their appearance. The main ingredients of the essential oil of NS were assessed using high-performance liquid chromatography (HPLC) (SPD-10AVP; Shimadzu, Kyoto, Japan) with the ODS column by a phytochemical specialist.
Experimental protocol: Based on the blood pressure value measured 14 days before the trial, subjects were randomly assigned to three groups, i.e. placebo and 100- and 200-mg test groups.
The case groups received extract of NS as two capsules per day containing 100 and/or 200 mg of the drug. At baseline (day 0) and at week 8, the body weights were measured, and fasting blood samples were collected after blood pressure measurement and subjected to hemato- logical evaluation (white blood cells, red blood cells, platelets, hematocrit, and hemoglobin) and blood chem- ical tests [i.e. aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehy- drogenase, blood urea nitrogen, serum creatinine, uric acid, total protein, albumin, total cholesterol, high- density-lipoprotein (HDL)-cholesterol and low-density- lipoprotein (LDL)-cholesterol].
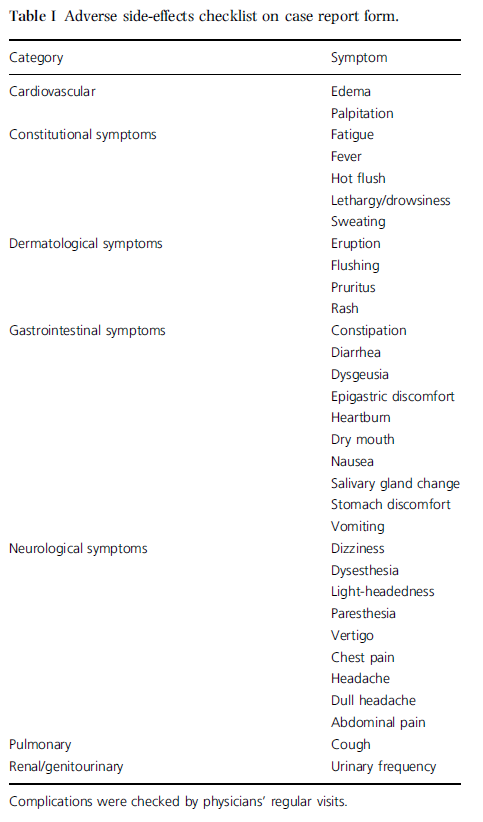
Adverse side-effects were checked by physicians’ interview using a case-report form at baseline (day 0), and at weeks 4 and 8 (Table I). Subjects were also asked not to change their dietary habits during the study, particularly with regard to their consumption of fat, salt, vegetables, and fruits.
Blood pressure measurement: Blood pressure and pulse were measured 14 days before the trial, at baseline (day 0), and 4 and 8 weeks after the start of the study under the morning fasting condition (12 ± 2 h after the last drug ingestion). Three measurements with an interval of 5 min were taken in the right arm using an automated sphygmomanometer (HEM- 707; Omron Co, Kyoto, Japan) and an appropriate-sized cuff after at least 10 min of rest in the seated position. The median of three measurements was taken as the measured value.
Statistical analysis: All values were expressed as mean ± SEM. The characteristics of groups at baseline were compared by one-way analysis of variance (ANOVA). The changes in blood pressures, pulse, and body weight were analyzed using one-way repeated-measures ANOVA and Tukey–Kramer post hoc method and their comparison with the baseline data. A P-value of less than 0.05 was considered to indicate a statistical significance. Statistical analyses were performed using SPSS statistical software (version 9.5).
RESULTS
The main constituents of the essential oil of NS were cymenen P (41.5%), terpinene G (13%), campholenel a (9.8%), thujene a (7.8%), pinene b (2.9%), limonene (2.9%), pinene a (2.2%), carvacrol (2.2%) thymoquinone (2.1%), and terpinene a (1.7%).
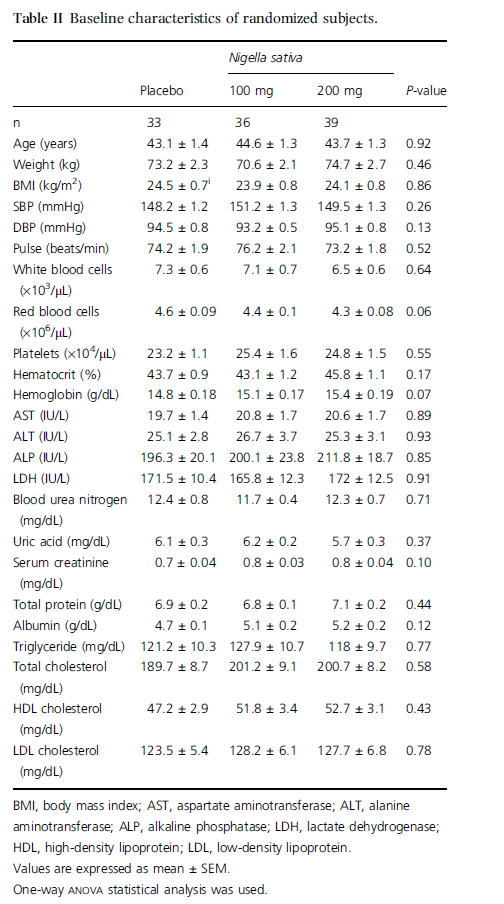
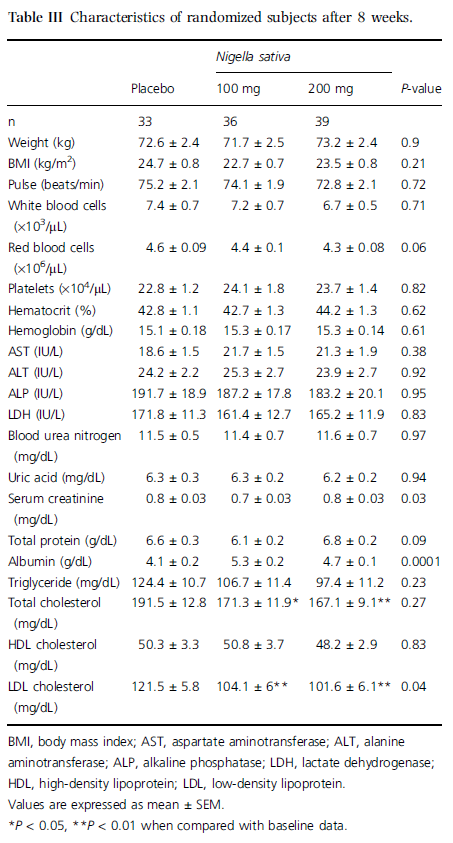
One hundred and nineteen applicants were enrolled for the study and 11 were excluded based on the aforementioned exclusion criteria. Thus, a total of 108 subjects were randomized and completed the study. At baseline, there were no significant differences among the three groups regarding blood pressure, pulse, age, weight, body mass index (BMI), and blood components (Table II). Four subjects failed to ingest the administered drug twice and one subject failed to ingest it for 3–4 days; thus, 103 subjects ingested 100% of the doses of the medicine. In our study, no complications in the test or placebo groups were observed on clinical and physical examination according to defined criteria. The SBPs of test groups (i.e. NS 100 and NS 200) were significantly lower than the baseline level (P = 0.04 and 0.009 after 4 weeks; P = 0.03 and 0.008 after 8 weeks of NS administration, respectively). Meanwhile, no significant change in SBP was observed in the placebo group when compared with baseline values (Figure 1). The DBP of the placebo group was not significantly changed after 4 and 8 weeks (P = 0.31 and 0.22, respectively) when compared with baseline, whereas those of the test groups showed significant decreases (P = 0.008 and 0.007 for NS 100 and NS 200, respectively) when compared with baseline data (Figure 1).
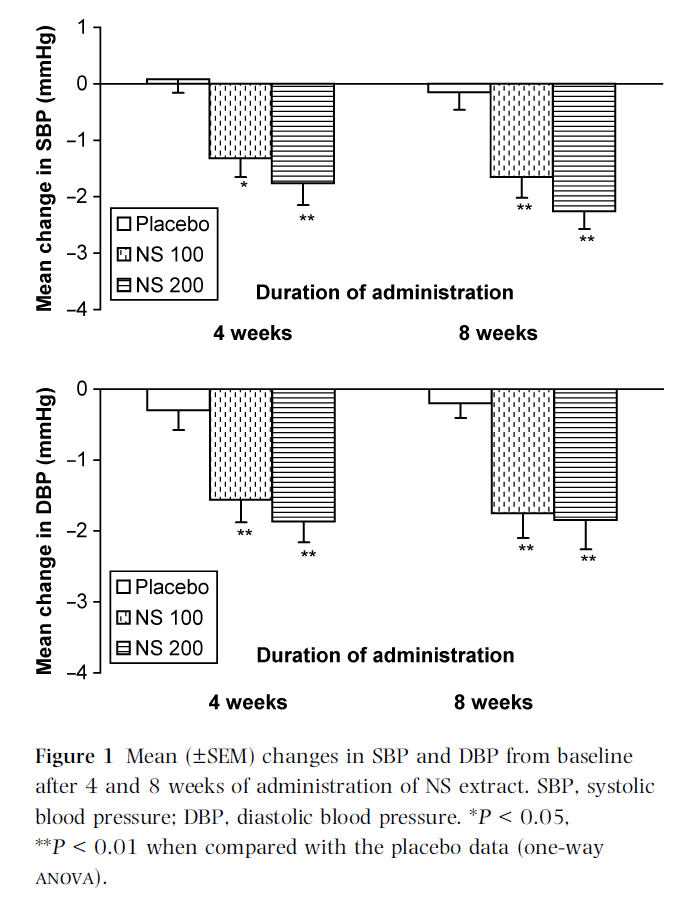
No significant changes in body weight or pulse from baseline were observed in the two test groups or placebo groups or among the two test groups (Table III). Regarding serum lipids, there was a significant reduction in total (P < 0.05–0.01) and LDL-cholesterol (P < 0.01) after 8 weeks of NS administration in test groups when compared with baseline data. There was also no such change in the placebo group (Table III).
DISCUSSION
In the present study, the administration of NS seed extract for 8 weeks reduced SBP and DBP in a dose-dependent manner and lowered the levels of total and LDL- cholesterol in patients with mild HT. Research reports indicate a variety of effective alternative therapies for alleviating high blood pressure including diet, exercise, herbs, and so forth. We particularly selected nonsmoking, recently diagnosed patients with mild HT, receiving no antihypertensive or lipid-lowering pharmaceutical therapy, with no significant cardiovascular risk factors other than HT. Patients who reported taking vitamins and other food additives were also excluded from the trial, avoiding the potential bias arising from recruiting participants who are more health-conscious than average. The reduction of blood pressure observed in our study was accompanied by significant changes in plasma concentrations of total and LDL-cholesterol. Participants were strictly advised and repeatedly reminded to maintain their current diet and lifestyle. Thus, our results are unlikely to be attributed to lipid- or weight-lowering regimens, dietary changes, or enhanced physical activity. Although we did not measure indices of the antioxidant defense system and lipid peroxidation, our assumption is that the reduction in blood pressure observed in our study was in part due to the antioxidant activity of the NS extract. The essential oil of NS seed has antioxidant property that makes it useful for treating cardiovascular disorders [16–18]. Some researchers have demonstrated a link between the administration of antioxidants or their respective plasma levels and the development of HT, atherosclerosis, and cerebral stroke [19–23]. Our results are consistent with those examining the effects of vitamins, natural products, or dietary modifications on cardiovascular morbidity, blood pressure, and antioxidant activity [24–26]. In a clinical trial, an increase in consumption of dietary fruits and vegetables, for an 8-week period reduced SBP and DBP by nearly 2.8 and 1.1 mmHg, respectively, more than those receiving control diet [27]. In the present study, NS seed extract capsules were used. In these capsules, concentrations of various bioactive substances were known and standardized, providing a standard intervention for all subjects. Standardization of treatment enables researchers to better monitor patients’ compliance to therapy and establish more accurate dose-response relations. Ease of administration and lack of side-effects are some of the main characteristics of natural products such as those used in this study. It is a well-known fact that lack of compliance, in part due to side-effects of antihypertensive drug therapy, is an important cause of treatment failure in hypertensive patients [28]. The positive results in our pilot trial are encouraging. However, our relatively small study population comprised low-risk patients with mild HT who were naive to drug therapy. The course of therapy in our study was relatively short, and it is not clear whether the beneficial effect of NS extract will persist with prolonged administration. The treatment in our trial was a primary therapeutic intervention and it is reasonable to assume that these low-risk patients who have only minor or no vascular damage will be more responsive to the intervention than patients with moderate to severe HT and with more advanced vascular disease and on multiple drug therapy. Reduction in blood pressure from mild HT range to high normal range, such as achieved in our pilot study, is clinically considerable. Maintaining patients in normotensive state and preventing their progression to higher- grade HT may postpone or even eliminate the need for antihypertensive drug therapy. Studies with larger and more diverse populations examining the antihypertensive effect for longer periods are warranted to establish the role of this extract as an antihypertensive agent. This study was also limited by the lack of availability of measures of endothelial function and oxidative stress in the studied groups. In addition, self- reported data for medication use, exercise, and diet information may not be as precise as expected.
In this study, a significant reduction in total cholesterol and LDL-cholesterol were also observed following NS consumption that may be useful in the long term to lower risk factors of cardiovascular disorders. It has previously been reported that hypertensive patients are likely to benefit from therapeutic interventions targeted at other modifiable risk factors. In particular, the high prevalence of coexisting HT and dyslipidemia suggests that many hypertensive patients might benefit from lipid-lowering therapy. This implies that a combined approach to reducing blood pressure and cholesterol could be twice as beneficial as targeting just one of these risk factors [29,30]. To conclude, this study demonstrated that the daily intake of NS seed extract could reduce DBP and SBP that may help prevent stroke and coronary heart disease in hypertensive subjects.
REF E REN C ES
- Domanski M., Mitchell G., Pfeffer M. et al. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA (2002) 287 2677–2683.
- Whelton P.K., He J., Appel L.J. et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA (2002) 288 1882–1888.
- Japanese Society of Hypertension Guidelines Subcommittee for the Management of Hypertension. Guidelines for the manage- ment of hypertension for general practitioners. Hypertens. Res. (2001) 24 613–634.
- FitzGera`ld R.J., Murray B.A., Walsh D.J. Hypotensive peptides from milk proteins. J. Nutr. (2004) 134 980S–988S.
- Ferrari C.K. Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology (2004) 5 275–89.
- Yao L.H., Jiang Y.M., Shi J et al. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. (2004) 59 113–122.
- Mills S. Monitoring herbal safety: current debate and resources. Symposium Report: Pharmacovigilance of Herbal Medicines: Current State and Future Direction, London, 24–26 April 2006. Phytomedicine (2007) in press.
- Ali B.H., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. (2003) 17 299–305.
- Bamosa A.O., Ali B.A., Sowayan S.A. Effect of oral ingestion of Nigella sativa seed on some blood parameters. Saudi Pharm. J. (1997) 5 126–9.
- Bamosa A.O., Basil A.A., Zubaida A.A. The effect of thymoquinone on blood lipids in rats. Indian J. Pharmacol. (2002) 46 195–201.
- Kanter M., Meral I., Dede S. et al. Effects of Nigella sativa L. and Urtica dioica L. on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in CCl4-treated rats. J. Vet. Med. A. Physiol. Pathol. Clin. Med. (2003) 50 264–8.
- Mahfouz M., El-Dakhakhny M. Some chemical and pharmacological properties of the new anti-asthmatic drug, Nigellone. Egypt. Pharm. Bull. (1960) 42 411–24.
- Toppozada H.H., Mazloum H.A., El Dakhakhny M. The antibacterial properties of the Nigella sativa l. Seeds. Active principle with some clinical applications. J. Egypt. Med. Assoc. (1965) 48 (Suppl-202) 187–202.
- el Tahir K.E., Ashour M.M., al Harbi M.M. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen. Pharmacol. (1993) 24 1123–31.
- Zaoui A., Cherrah Y., Lacaille-Dubois M.A., Settaf A., Ama-rouch H., Hassar M. Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat. Therapie (2000) 55 379–82.
- Ghosheh O.A., Houdi A.A., Crooks P.A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J. Pharm. Biomed.
- Mansour M.A., Ginawi O.T., El-Hadiyah T., El-Khatib A.S., Al-Shabanah O.A., Al-Sawaf H.A. Effects of volatile oil constit- uents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: evidence for antioxidant effects of thymoquinone. Res. Commun. Mol. Pathol. Pharmacol. (2001) 110 239–51.
- Eichholzer M., Stahelin H.B., Gey K.F. Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke. EXS (1992) 62 398–410.
- Gey K.f., Stahelin H.B., Eicholzer M. Poor plasma status of carotene and vitamin C is associated with a higher mortality from ischemic heart disease and stroke: basal prospective study. Clin. Invest. (1993) 71 3–6.
- Redon J., Oliva M.R., Tormos C. et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension (2003) 41 1096–1101.
- Noguchi T., Ikeda K., Sasaki Y. et al. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Hypertens. Res. (2001) 24 735–742.
- Mullan B.A., Young I.S., Fee H., MaCance D.R. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension (2002) 40 804–809.
- Rimm E.B., Stampfer M.J., Ascherio A., Giovannucci E., Colditz G.A., Willett W.C. Vitamin E consumption and the risk of coronary heart disease in men. N. Engl. J. Med. (1993) 328 1450–6.
- Stephens N.G., Parsons A., Schofield P.M., Kelly F., Cheeseman K., Mitchinson M.J. Randomized controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet (1996) 347 781–6.
- Stein J.H., Keevil J.G., Wiebe DA., Aeschlimann S., Folts J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation (1999) 100 1050–5.
- Appel L.J., Moore T.J., Obarzanek E. et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. (1997) 336 1117–24.
- Andrade J.P., Vilas-Boas F., Chagas H., Andrade M. Epidemiological aspects of adherence to the treatment of hypertension. Arq. Bras. Cardiol. (2002) 79 375–84.
- Wong N.D., Lopez V., Tang S., Williams G.R. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am. J. Cardiol. (2006) 98 204–8.
- Johnson M.L., Pietz K., Battleman D.S., Beyth R.J. Prevalence of comorbid hypertension and dyslipidemia and associated car- diovascular disease. Am. J. Manage. Care. (2004) 10 926–32. Anal. (1999) 19 757– 762.
- Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. (2000) 14 323–328.

