Promise Madu Emeka, Lorina Ineta Badger-Emeka,1Chiamaka Maryann Eneh,2 and Tahir Mahmood Khan3
Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Hofuf, Kingdom of Saudi Arabia1
Department of Biomedical Sciences, College of Medicine, King Faisal University, Hofuf, Kingdom of Saudi Arabia2
Department of Microbiology, University of Nigeria Nsukka, Nsukka, Nigeria3
Abstract
Background:
The aim of the study was to investigate the effects of dietary combination of Nigella sativa seed and oil extracts with chloroquine (CQ), and how these combinations enhance CQ efficacy in mice infected with Plasmodium berghei and their survival rates.
Materials and Methods:
Chloroquine sensitive P. berghei, NK65 strain was used for the study. This was passaged intraperitoneally into albino mice with a 0.2ml standard inoculum consisting of 106 parasitized erythrocyte suspension in phosphate buffer solution (PBS). Parasitaemia was ascertained by microscopical examination of blood films under oil immersion at X100 magnification.
Results:
Nigella sativa seed in feed (NSSF), NSSF + CQ on day 4, produced 86.1% and 86.0% suppression respectively, while Nigella sativa oil extract in feed (NSOF) and in combination with CQ had 86.0% and 99.9% suppression respectively. The degree of suppression with the combination was significantly higher compared to CQ alone (P < 0.001) (36.1%). Complete parasitaemia clearance was obtained on the 20th and 15th day of treatment for NSSF, NSSF + CQ respectively, while that for NSOF and NSOF + CQ was on days 26 and 12 respectively. For CQ parasite clearance was 12 days with treatment. Also, the combinastion of 10 mg/kg Nigella sativa oil treatment injected intraperitoneally with oral CQ produced very significant parasite suppression (P < 0.0001) (93%). Survival rate in NSSF and NSOF and in combination with CQ groups was 100 and 60.0% for CQ alone.
Conclusions:
This study shows that the use of Nigella sativa seed and oil extract as dietary supplements in combination with CQ has a potential in enhancing the efficacy of CQ and could be of benefit in management of malaria.Keywords: Chloroquine, nigella sativa, parasitaemia, plasmodium berghei, suppression
INTRODUCTION
Malaria remains a public health problem in the tropics and sub Saharan regions of the world with Africa carrying the major burden of the disease. Significant cases have been reported in Asia, the Pacific, America, Middle East and some parts of Europe.[1] It is estimated that 216 million cases occurred globally in 2010,[2] with about 655,000 deaths. Chloroquine is still considered the drug of choice due to its cheap cost and availability compared to other anti-malaria drugs.[3] Morbidity and mortality observed in cases of malaria are attributable to toxicity and emergence of resistance to antimalarials.[4] This situation has given rise to co-administration with supplements to reduce the effects of both the drugs and disease particularly in children and pregnant women.[5,6] According to reports, chloroquine and malaria are known to cause oxidative stress.[7] Complementary use of antioxidants of natural origin with conventional antimalarial like chloroquine has been used in the past, particularly with those whose safety and efficacies are known and are employed in the treatment of malaria as an adjunct.[8,9,10] The use of an antioxidant will undoubtedly reduce the oxidative stress caused by parasite and chloroquine. Hence, improving host antioxidant status will help to decrease parasite load by suppressing parasite multiplication. Also, this action will be crucial in advancing the acquisition of a natural immunity of the host and help to eradicate completely the remaining invading parasites with or without drugs.[11]
Nigella sativa (family Ranuculaceae) commonly known as the Black cumin is grown in most Middle East and Far East countries were the seeds and oil are used as natural remedies, condiments/spices in food products.[12] Documentary evidence shows that this ancient traditional remedy can be used in combination with conventional drugs in various disease conditions because of its antioxidant properties.[13] Past studies used different extracts of various fractions of Nigella sativa to demonstrate its antimalarial activity in rodents,[14] however micronutrients which potentiate the effects of major components would have been lost during extraction. Literature is however silent on the dietary supplementation with Nigella sativa in malaria treatment. The present study evaluates the efficacy of dietary supplementation with seed and oil extract of Nigella sativa in mice infected with Plasmodium berghei. The author also looked at the effects of combining dietary supplements of N. sativa seeds and oil extract with chloroquine on parasite clearance and survival rate.
MATERIALS AND METHODS
Nigella sativa seed and oil preparation
Dried Nigella sativa (black cumin) seeds of Yemeni origin were bought from a local traditional medicine shop at Hofuf, Al Ahsa, Kingdom of Saudi Arabia. They were identified by a pharmacognosist in King Faisal University, Saudi Arabia. The seeds were crushed into powder using a ceramic mortar and pestle and then incorporated with animal feed at a ratio of 1:5. This mixture was termed Nigella sativa seed in-feed (NSSF).
The black seed oil was also bought from the same shop, a product of Al-Hussan Food Products Factory, Riyadh. According to manufacturer guideline, it is cold press extracted 100% pure organic oil. It was also incorporated in animal feed in a ratio of 1:5 labeled Nigella sativa oil in-feed (NSOF). Also, the oil extract was diluted in 3% dimethyl sulphoxide (DMSO) to obtain a working concentration of 500 mg/ml and used as 5 mg/kg and 10 mg/kg respectively.
Preparation of Chloroquine phosphate solution
Chloroquine phosphate 250 mg by Evans Medical PLC, Ogun State, Nigeria were used for the experiments. This was dissolved in distilled water to obtain a final dose of 10 mg/kg body weight.
Animals and Parasite strain
Male and female (non-pregnant) albino mice with a mean weight of 22.18 ± 2.2 were used for this study. They were obtained from the animal house of the college of Vet Medicine of University of Nigeria, Nsukka and maintained there during the study in line with the university’s ethical policy on the use of animals. The animals were kept in groups in well ventilated cages in accordance with the internationally accepted principles for laboratory animal use and cares as found in the National Institute of Health Guidelines for care of laboratory animals.[15] They were fed on mice feed diet and with water ad libitum. Animals were acclimatized for two weeks before the start of the experiment.
Plasmodium berghei was used for the study. They were chloroquine sensitive NK 65 strain obtained from the Biochemistry Department, Nigerian Institute for Medical Research, Yaba-Lagos, Nigeria. The parasites were then maintained in fresh animals by serial passaging of blood from a donor mouse obtained from the tail vein which was suspended in phosphate buffer solution (PBS).
Parasite inoculum and determination of the course of infection
Parasitaemia was established microscopically. The number of erythrocytes per micro litre of blood was calculated using a Neubear Haemocytometer,[16] with each mouse infected with 0.2ml standard inoculum of 106 parasitized erythrocyte suspension in PBS intraperitoneally. The course of infection for all experiments was monitored daily. Parasitaemia was ascertained daily by microscopically examining thin blood films prepared from blood obtained from the tail vein of infected mice and calculated as the percentage of infected erythrocytes in fields of 500 erythrocytes. In vivo antimalaria activity against P. berghei was done according to Rane’s as described by Elufioye and Agbedahunsi.[17]
Experimental protocol
The study was conducted in two phases. In the first phase, animal feed was supplemented with both Nigella sativa seed and 100% oil extract. Also both were combined with chloroquine (CQ) in different study groups. For these experiments, animals were selected randomly and placed in 4 groups consisting of 5 (Five) mice per treatment group. Group 1 consisted of Nigella sativa seed plus feed (NSSF), while group 2 represented the mice given NSSF + CQ. The second set in phase 1consisted of group 3 mice that were administered with Nigella sativa oil extract in feed (NSOF) and group 4 that received NSOF + CQ in combination. In the phase 2 experiments, mice were placed in 4 groups consisting of 5 mice each. Groups 1 and 2 were given 5 mg/kg, 10 mg/kg doses of Nigella sativa oil extracts respectively which has been diluted in 3% DMSO. These represented 0.22 ml and 0.44 ml of 500 mg/ml working concentration according to the method of Mansour et al.,[18] but slightly modified to accommodate the two dose levels. The remaining mice were put in groups 3 and 4 in which they were given a combination of 5 mg/kg + CQ and 10 mg/kg + CQ respectively.
The suppressive treatment study comprised of six groups of five mice each, namely NSSF, NSOF, NSSF plus CQ, NSOF plus CQ, 5 mg/kg and 10 mg/kg doses of Nigella sativa oil extract groups. Mice were exposed to their different treatments 72 h before infection.
Control experiments had groups of fifteen mice divided into five each. In the first control group, mice were infected with P. berghei and were not given any treatment hence they represented untreated infected control (UTIC) group. They were however given 0.2mls PBS orally. The second control group was termed the positive control in which mice were inoculated with P. berghei and oral chloroquine (CQ) treatment (10 mg/kg) commenced immediately after parasite inoculation. The last control group was infected with P. berghei and treated with 0.2mls of 3% DMSO orally. Oral gavage was by the aid of oral cannula.
For all the groups, tail blood films were prepared starting from day 3 post parasite inoculation and on daily basis till day 16 post inoculation and thereafter, every other day until the experiment was terminated. The time of parasite clearance was determined and this was defined as the time of disappearance of peripheral parasitaemia in the infected mice following treatment. All the experimental groups were monitored post parasite disappearance for any reappearance of parasitaemia during the follow-up period. The number and percentage of survivors by days was also recorded. Suppressive effects were monitored after 4 days post parasite inoculum, according to the method described by Peters et al.,[19] Observation and feeding continued until parasitaemia cleared completely. The average percentage parasitaemia suppression was calculated according to the method of Peters and Robinson,[20] using the formula by Gessler et al.,[21] 100-[(mean parasitaemia treated/mean parasitaemia control) ×100].
Data analysis
Data is hereby presented as mean ± SD then analysed statistically using IQ Macros 2013 software in Microsoft Excel®. One-way ANOVA test was used to determine levels of significance, then Turkey honestly significant difference [HSD] post-hoc test to confirm significance between groups. P < 0.001 was considered to be significant.
RESULTS
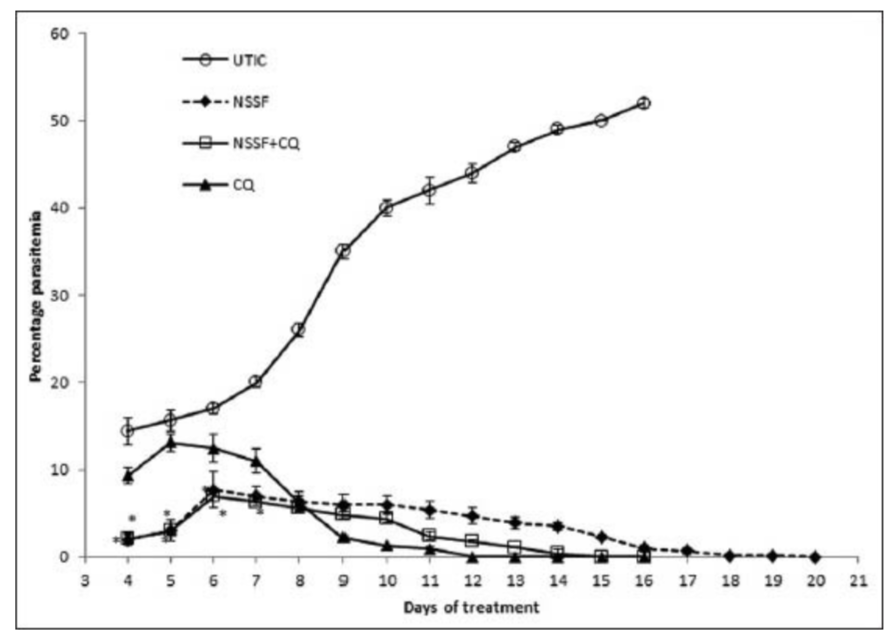
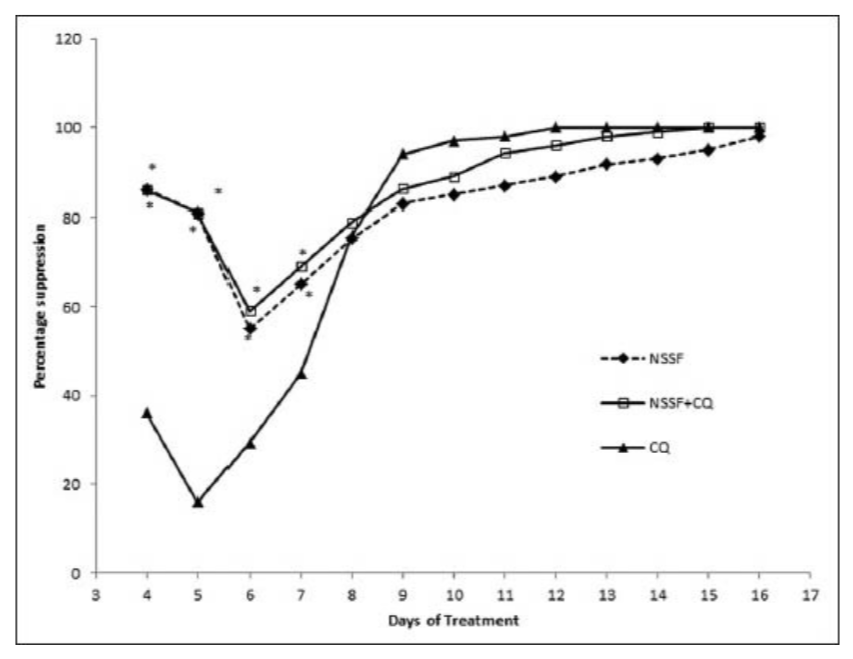
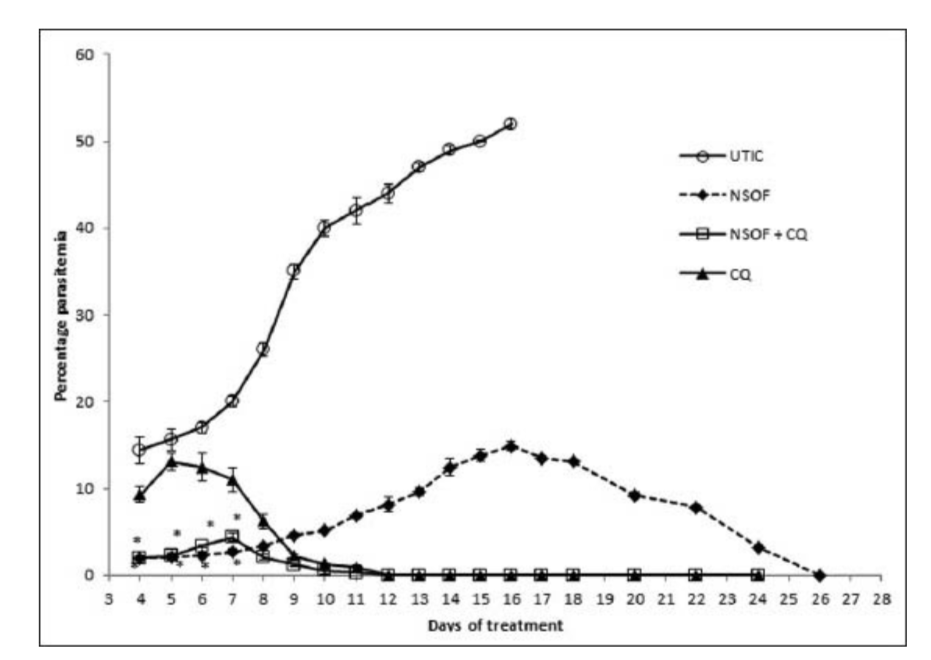
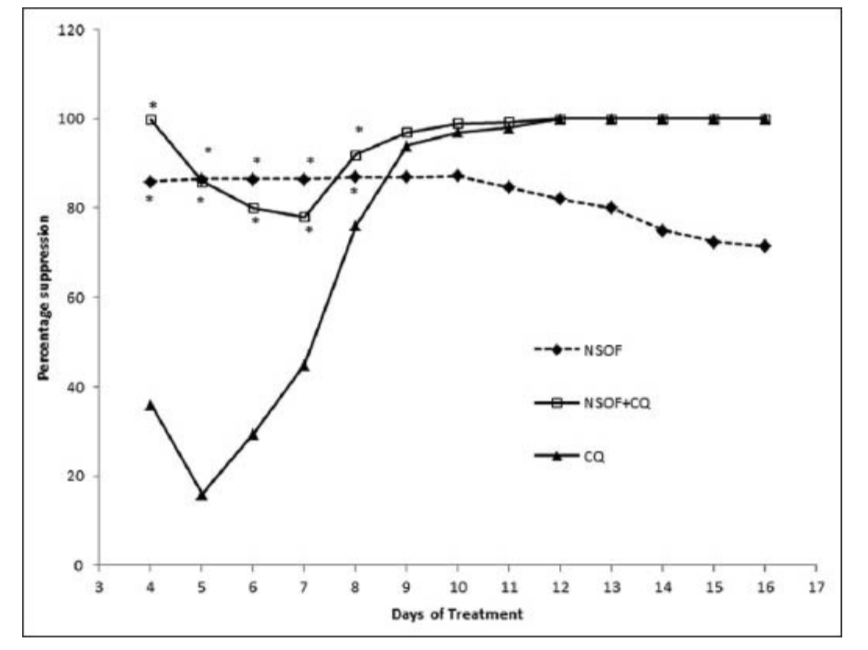
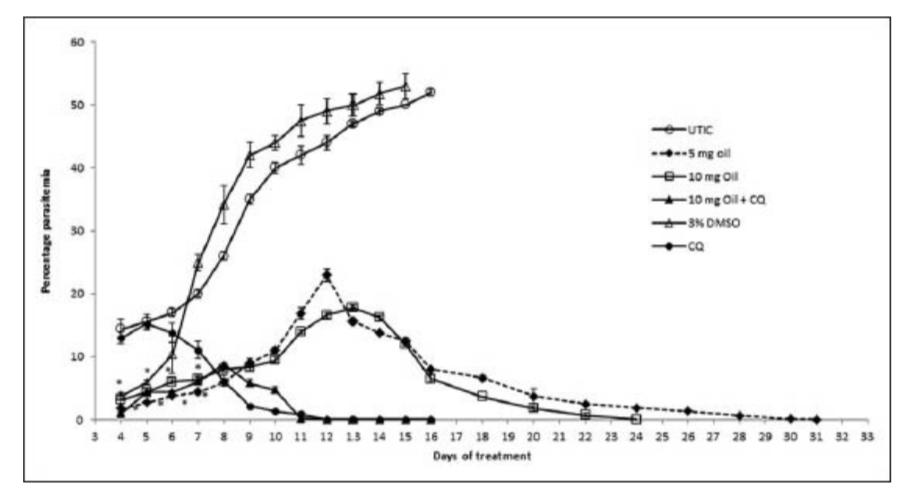
A progressive increase in mean percentage parasitaemia was observed until day 6 in the groups treated with NSSF and NSSF + CQ as presented in Figure 1. However, CQ alone peaked on day 5 with the highest mean percentage parasitaemia of 13.1% ±1.0. This was followed by a parallel decrease in mean percentage parasitaemia for all the treatment groups until clearance. Parasitaemia did not clear for NSSF until day 20 but for NSSF + CQ it cleared on day 15, whereas CQ alone cleared after 12 days. Percentage suppression on day 4 was highest for NSSF with 86.1%, followed by NSSF + CQ with 86% which were very significant (P < 0.001), compared to CQ (36.1%) as shown on Figure 2. Results show that up till day 8 post infection, treatment with NSSF and NSSF + CQ had better suppressive effects on parasitaemia than CQ. Compared with the (UNTIC) untreated infected control, both suppression and parasite clearance were very significant (P < 0.0001), showing definite anti-plasmodial activity. Results of supplementation with NSOF revealed a gradual increase in parasitaemia which peaked on day 16 and eventually cleared on day 26 [Figure 3]. The mice in this group appeared very agile with good skin colour, good appetite and no sign of morbidity. However, when combined with CQ the result was better as parasitaemia cleared within 12 days, same as chloroquine. In this group (NSOF + CQ)% suppression was higher than with chloroquine alone with a 99.9% at 4 days post infection and the results were significant (P < 0.0001) as shown in Figure 4. Results on mean percentage parasitaemia in mice treated with 5 mg/kg, 10 mg/kg Nigella sativa oil extract and in combination with CQ are shown in Figure 5. The 10 mg/kg oil group showed a better parasitaemia clearance than those treated with 5 mg/kg oil. However, both treatments took a longer time before parasite clearance was observed as compared to that of CQ alone. Combining 10 mg/kg oil extract with CQ showed a faster parasite clearance than the CQ treated group. Four day suppressive observation showed 88, 78, 93 and 0% for 5 mg/kg, 10 mg/kg, 10 mg/kg + CQ and CQ respectively and the results are presented in Figure 6. Again, combination with CQ produced a greater parasite suppression compared with either oil extract or CQ alone. This therefore implies that Nigella sativa seeds did have suppressive effect on P. berghei infected mice. When the seed or oil was combined with chloroquine, a better suppressive effect was obtained. Our results also revealed that animal survival was affected by the various treatments. There was 100% survival amongst P. berghei infected mice treated with NSSF, NSOF and in combination with CQ, until the experiments were terminated 45 days later. Treatment with CQ had 60% survival rate while 3% DMSO control and UTIC had no survivors.

DISCUSSION
The challenges in looking for effective malarial treatment will continue until patients begin to get relief from the infection. Research in the area of the use of supplements with conventional antimalarial drugs is not new[5,6] but has begun with traditional remedies in the light of ineffectiveness of existing mode of treatment. Traditional use of remedies such as Nigella sativa as adjuvant is becoming popular as some of them are common knowledge and have been employed for centuries in the treatment of different diseases.
The findings from the present study showed that there was an earlier parasite clearance with either NSSF or NSOF when combined with CQ than in CQ treatment alone. This shows that N. sativa seed or oil extracts as supplements enhanced parasite clearance when combine with CQ. Methanol and ethanol extracts of N. sativa have been demonstrated to have anti-plasmodia activity in mice infected with animal species of the plasmodium.[14,22,23] Reports indicate that N. sativa acts as an antioxidant by reducing the production of free radicals, hence by this action it augments the antioxidant status of the host, enabling the host to reduce the effects of reactive oxygen species and therefore decreases oxidative stress generated by malaria and in the cause of treatment with chloroquine.[23] This might explain the complete parasite clearance as seen in some of the groups during the present investigation. Treatment of malaria in Nigeria particularly in this era of drug resistance is expensive for a majority of the sufferers who might barely afford the antimalarial drug designated as first-line and will not see the need to buy any prescribed antioxidant as they are added expenses. Ekeanyanwu et al.,[24] and Aghedo et al.,[25] showed from their studies that the antioxidant levels in plasmodium-parasitized children in the North-West of Nigeria were low and that the more severe the malarial infection, the lower the antioxidant level and the packed cell volume. They recommend that malaria-parasitized children, particularly those in the North-West of Nigeria, be placed routinely on antioxidant vitamins to manage the micronutrient deficiencies as was seen in the children. The non-usage of antioxidants to manage micronutrient deficiencies is not limited to one geographical region according to an earlier report by Ekeanyanwu et al.,[24] from studies carried out in Southeastern Nigeria. Therefore, there will be potential benefits of antioxidant supplementation with the use of CQ or other antimalarials.[7] We also observed that complementary use N. sativa with CQ produced enhanced parasite suppression which was very significant. In comparison, NSOF is seen to have produced earlier parasite suppression than NSS treatment group with this effect further improved with the addition of CQ. This potentially makes the Nigella sativa oil extract a more likely antimalarial agent as an adjuvant. The present finding is consistent with the work of Dwived et al.,[10] in which they reported that the co-administration of CQ and an immune stimulant Picrurhiza kurrua extract enhanced the efficacy of CQ in murine mice. Also, studies of co-administration of various antimalarial tested medicinal plant extracts in Madagascar and Kenya revealed a reversal in chloroquine resistance in animals.[26,27] In other studies, diet supplementation with genisten was reported to have suppressed liver infection with P. berghei thus reducing the blood parasite load.[28] It is important to note that the liver stage is the rate-limiting step and critical for the subsequent erythrocyte stage. The arrest of the hepatic parasitic stage is usually targeted for prophylaxis since it will stop parasite multiplication. As studies have shown that Nigella sativa (seed and oil) will enhance the antioxidant profile,[29,30] it will also assist the host to build up natural immunity needed to fight the clinical stage of the infection. In combination with an antimalarial agents such as CQ, the effect will be the much-needed cure as is shown by the results in the present study. Malaria is still claiming casualties because of difficulties in its eradication; it is not surprising that several treatment options have been advocated by both orthodox and traditional practitioners in this fight. The life expectancy of the average Nigeria has dropped considerably over the years with the emergence of chloroquine resistant Plasmodium parasites.[31] Due to multidrug resistance associated with the use of antimalarials, supplementation and/or combination with CQ have been tried.[32] Nigella sativa seed and oil as shown in this study have the potential to be used as both supplementation and as adjuvant. It is of great significance that the results from the present study showed that either the seed, oil or in combination with CQ produce no mortality and therefore safe.
CONCLUSION
This study has further highlighted the therapeutic potential of this plant seed extract as a medicinal supplement. In malarial endemic area, the use of Nigella sativa as an adjuvant will reduce the adverse effect of CQ and the cost of malarial treatment with CQ being the cheapest and most available.
Reference
Emeka, Promise Madu et al. “Dietary supplementation of chloroquine with nigella sativa seed and oil extracts in the treatment of malaria induced in mice with plasmodium berghei.” Pharmacognosy magazine vol. 10,Suppl 2 (2014): S357-62. doi:10.4103/0973-1296.133282
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4078332/

