Md. Asaduzzaman Khan1, Han-chun Chen1*, Mousumi Tania1 and Dian-zheng Zhang1,2
1-Department of Biochemistry, School of Biological Science and Technology, Central South University, Changsha, Hunan 410013, P R China.
2-Department of Biochemistry/Molecular Biology, Philadelphia College of Osteopathic Medicine, Philadelphia, PA 19131, USA
*E-mail: chenhanchun@mail.csu.edu.cn, asadkhanbmj@yahoo.com
Abstract
Nigella sativa has been used as traditional medicine for centuries. The crude oil and thymoquinone (TQ) extracted from its seeds and oil are effective against many diseases like cancer, cardiovascular complications, diabetes, asthma, kidney disease etc. It is effective against cancer in blood system, lung, kidney, liver, prostate, breast, cervix, skin with much safety. The molecular mechanisms behind its anticancer role is still not clearly understood, however, some studies showed that TQ has antioxidant role and improves body’s defense system, induces apoptosis, and controls Akt pathway. Although the anti-cancer activity of N. sativa components was recognized thousands of years ago, but proper scientific research with this important traditional medicine is a history of the last 2~3 decades. There are not so many research works done with this important traditional medicine and very few reports exist in the scientific database. In this article, we have summarized the actions of TQ and crude oil of N. sativa against different cancers with their molecular mechanisms.
Keywords: Traditional medicine, Nigella sativa, Thymoquinone, Antioxidant, Anti-cancer mechanism
Introduction
Cancer is one of the major threats of modern life, which is considered as the second cause of death after myocardial infarction (Grundy, 1991). Millions of people die every year in different types of cancer despite tremendous efforts to find methods of control and cure. In the last century, great advances were made in modern medical science to control the disease. But many diseases like cancers are not yet curable fully. To find out new and authentic therapies, scientists are working with traditional or folk medicines in parallel with modern medicine. Nigella sativa has been used for medicinal purposes for centuries. It originated from Southeastern Asia and also used in ancient Egypt, Greece, the Middle East, and Africa. In Islam, it is regarded as one of the greatest forms of healing medicine available (Nigella-sativa-research.com, 2010; Wikipedia, 2010). It is a flowering plant, of which seed is used as a spice. The seed is called black cumin in English, while in old Latin it was called ‘Panacea’ meaning ‘cure all’; in Arabic it is termed as ‘Habbah Sawda’ or ‘Habbat el Baraka’ translated as ‘Seeds of blessing’. It is also known as ‘Kalo jeera’ (in Bangladesh), ‘Kalonji’ (in India) and ‘Hak Jung Chou’ in (China) (Aggarwal et al., 2008). Both seeds and oil extracted from this plant are used in medicinal purposes. The active ingredients of N. sativa have beneficial effects against many diseases, including cancers. For example, it is effective in the diminishing the risk of atherosclerosis by decreasing the serum low density lipoprotein cholesterol level and increasing the serum high density lipoprotein cholesterol levels (Dahri et al., 2005; Nader et al., 2010); it exerts therapeutic and protective effect in diabetes by decreasing morphological changes and preserving pancreatic beta-cell integrity (Kanter et al., 2009) and by beneficially changing the hepatic enzyme activities (Pari and Sankaranarayanan, 2009); it is effective against hypertension (Khattab and Nagi, 2007; Dehkordi and Kamkhah, 2008); it has a potent antihistaminic effect on airways of asthmatic patients (Boskabady et al., 2010); its components are promising agents to complement schistosomiasis specific treatment (El Shenawy et al., 2008); its oil protects kidney tissue against oxygen free radicals, preventing renal dysfunction and morphological abnormalities (Bayrak et al., 2008; Uz et al., 2008; Ragheb et al., 2009). For thousands of year, the seeds, oils and extracts of N. sativa have been used as an anticancer agent by Unani, Ayurveda and the Chinese system of medicine that have originated from the Arab, Ind-Bangla and China, respectively. The modern scientific research with the investigation of anticancer activity of N. sativa is a comparatively recent affair (for the last 2~3 decades). There are not so many research works done in this field and very few review articles exist in this area. We have searched the scientific databases like Pubmed, Web of Science and Google scholar and summarized the current scientific information about the anticancer activities of N. sativa with mechanisms of action.
Role of N. sativa as an anticancer agent
Many active ingredients have been found in the seeds of N. sativa. The seeds contain both fixed and essential oils, proteins, alkaloids, and saponin (Ali and Blunden, 2003). Ghosheh et al. (1999) described the quantification of four
pharmacologically important components: thymoquinone (TQ), dithymoquinone (DTQ), thymohydroquinone (THQ), and thymol (THY), in the oil of N. sativa seed by HPLC. Much of the biological activities of the seeds have been shown to be due to thymoquinone, the major component of the essential oil, which is also present in the fixed oil (Ali and Blunden, 2003). TQ is considered as potent anti-oxidant (Badary et al., 2003), anti-carcinogenic and anti-mutagenic agent (Bourgou et al., 2008; Khader et al., 2010) (structure of thymoquinone is shown in Figure 1a). Moreover, TQ is a relatively safe compound, particularly when given orally to experimental animals (Al-Ali et al., 2008). Alpha (α)-hederin, a pentacyclic triterpene saponin (structure: Figure 1b) isolated from the seeds of N. sativa, was also reported to have potent in vivo antitumor activity (Swamy and Huat, 2003)
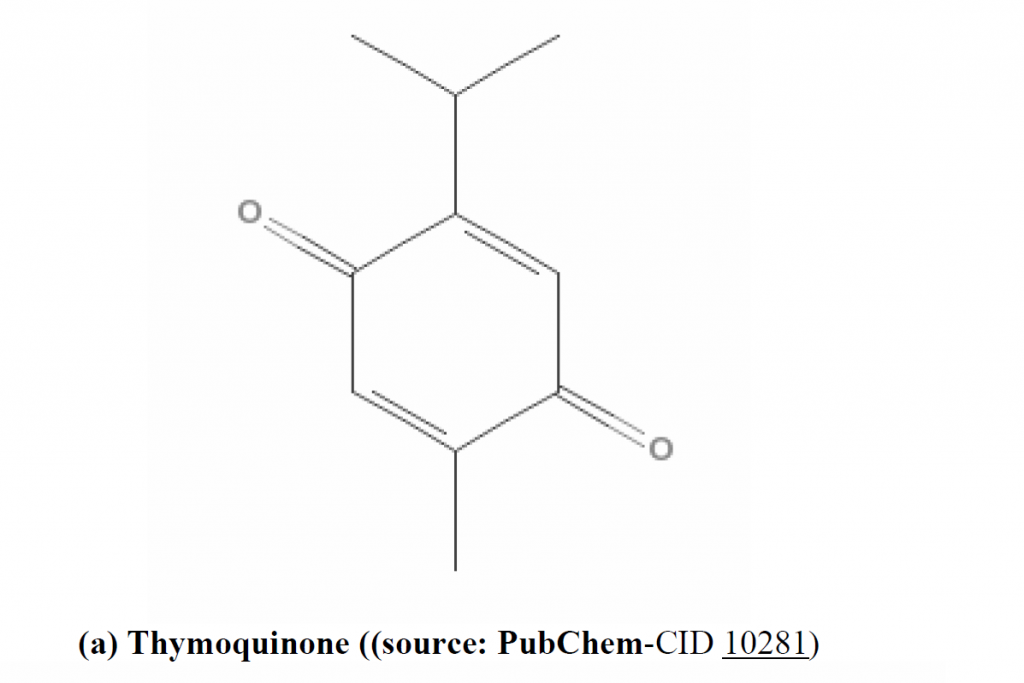
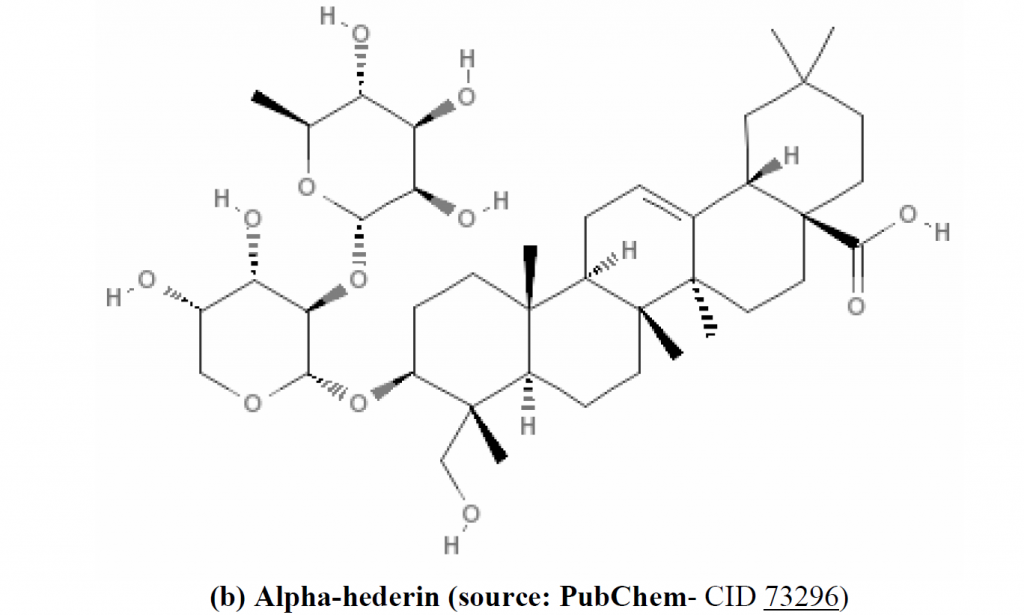
Figure 1. Chemical structure of anti-tumor agents isolated from N. sativa
N. sativa seeds or oils or its active ingredients like TQ are effective against different cancers:
Blood Cancer
El-Mahdy et al. (2005) reported that TQ exhibits anti-proliferative effect in human myeloblastic leukemia HL-60 cells. Derivatives of TQ bearing terpene-terminated 6-alkyl residues were tested in HL-60 cells and 518A2 melanoma by Effenberger et al. (2010). They found the derivatives induce apoptosis associated with DNA laddering, a decrease in mitochondrial membrane potential and a slight increase in reactive oxygen species. Swamy and Huat (2003) observed that α-hederin also induced death of murine leukemia P388 cells by a dose- and time-dependent increase in apoptosis.
Breast Cancer
Aqueous and alcohol extracts of N. sativa were found to be effective in vitro in inactivating MCF-7 breast cancer cells (Farah and Begum, 2003). N. sativa, in combination with melatonin and retinoic acid reduced the carcinogenic effects of DMBA (7, 12-di-methylbenz(a)anthracene) in mammary carcinoma of rats (El-Aziz et al., 2005). Terpene-terminated 6-alkyl residues of TQ were tested in MCF-7/Topo breast carcinoma by Effenberger et al. (2010). They found the derivatives inducing cell death by apoptosis.
Colon Cancer
Gali-Muhtasib et al. (2004) suggested that TQ is anti-neoplastic and pro-apoptotic against colon cancer cell line HCT116. Salim and Fukushima (2003) demonstrated that the volatile oil of N. sativa has the ability to inhibit colon carcinogenesis of rats in the post-initiation stage, with no evident adverse side effects. Norwood et al. (2006) suggested TQ as chemotherapeutic agent on SW-626 colon cancer cells, in potency, which is similar to 5-flurouracil in action. However, on HT-29 (colon adenocarcinoma) cell, no effect of TQ was found (Rooney and Ryan, 2005).
Pancreatic Cancer
Chehl et al. (2009) showed that TQ, the major constituent of N. sativa oil extract, induced apoptosis and inhibited proliferation in PDA (pancreatic ductal adenocarcinoma) cells. They also suggested TQ as a novel inhibitor of pro-inflammatory pathways, which provides a promising strategy that combines anti-inflammatory and proapoptotic modes of action. TQ also can abrogate gemcitabine- or oxaliplatin-induced activation of NF-kappa B, resulting in the chemosensitization of pancreatic tumors to conventional therapeutics (Banerjee et al., 2009). The high molecular weight glycoprotein mucin 4 (MUC4) is aberrantly expressed in pancreatic cancer and contributes to the regulation of differentiation, proliferation, metastasis, and the chemoresistance of pancreatic cancer cells. Torres et al. (2010) evaluated the down-regulatory effect of TQ on MUC4 in pancreatic cancer cells. But in a study, Rooney and Ryan (2005) did not find any preventive role of TQ on MIA PaCa-2 (pancreas carcinoma) cells.
Hepatic Cancer
The cytotoxic activity of N. sativa seed was tested on the human hepatoma HepG2 cell line by Thabrew et al. (2005), and 88% inhibitory effect on HepG2 was found after 24-hr incubation with different concentrations (0-50 mg/ml) of the N. sativa extract. Nagi and Almakki (2009) reported that oral administration of TQ is effective in increasing the activities of quinone reductase and glutathione transferase and makes TQ a promising prophylactic agent against chemical carcinogenesis and toxicity in hepatic cancer
Lung Cancer
Swamy and Huat (2003) mentioned the antitumor activity of α-hederin from N. sativa against LL/2 (Lewis Lung carcinoma) in BDF1 mice. Also, Mabrouk et al. (2002) showed that supplementation of diet with honey and N. sativa has a protective effect against MNU (methylnitrosourea)-induced oxidative stress, inflammatory response and carcinogenesis in lung, skin and colon. However, Rooney and Ryan (2005) reported that α-hederin and TQ, the two principal bioactive constituents of N. sativa enhance neither cytotoxicity nor apoptosis in A549 (lung carcinoma), HEp-2 (larynx epidermoid carcinoma) cells.
Skin cancer
Topical application of N. sativa extract inhibited two-stage initiation/promotion [dimethylbenz[a]anthracene (DMBA)/croton oil] skin carcinogenesis in mice. Again, intraperitoneal administration of N. sativa (100 mg/kg body wt) 30 days after subcutaneous administration of MCA (20-methylcholanthrene) restricted soft tissue sarcomas to 33.3% compared with 100% in MCA-treated controls (Salomi et al., 1991).
Fibrosarcoma
TQ from N. sativa was administrated (0.01% in drinking water) one week before and after MCA treatment significantly inhibited the tumor incidence (fibrosarcoma) and tumor burden by 43% and 34%, respectively, compared with the results in the group receiving MCA alone. Moreover, TQ delayed the onset of MCA-induced fibrosarcoma tumors. Also in vitro studies showed that TQ inhibited the survival of fibrosarcoma cells with IC50 of 15 mM (Badary and Gamal, 2001). Oil of N. sativa also decreased the fibrinolytic potential of the human fibrosarcoma cell line (HT1080) in vitro (Awad, 2005).
Renal Cancer
Khan and Sultana (2005) reported the chemo-preventive effect of N. sativa against ferric nitrilotriacetate (Fe-NTA)- induced renal oxidative stress, hyper-proliferative response and renal carcinogenesis. Treatment of rats orally with N. sativa (50 – 100 mg/kg body wt) resulted in significant decrease in H2O2 generation, DNA synthesis and incidence of tumors.
Prostate Cancer
TQ, from N. sativa, inhibited DNA synthesis, proliferation, and viability of cancerous (LNCaP, C4-B, DU145, and PC- but not non-cancerous (BPH-1) prostate epithelial cells by down-regulating AR (androgen receptor) and E2F-1 (a transcription
factor) (Kaseb et al., 2007). In this study, they suggested TQ as effective in treating hormone-sensitive as well as hormone- refractory prostate cancer. Yi et al. (2008) found that TQ blocked angiogenesis in vitro and in vivo, prevented tumor angiogenesis in a xenograft human prostate cancer (PC3) model in mouse, and inhibited human prostate tumor growth at low dosage with almost no chemotoxic side effects. Furthermore, they observed that endothelial cells were more sensitive to TQ-induced cell apoptosis, cell proliferation, and migration inhibition compared with PC3 cancer cells. TQ also inhibited vascular endothelial growth factor-induced extracellular signal-regulated kinase activation but showed no inhibitory effects on vascular endothelial growth factor receptor 2 activation.
Cervical Cancer
Shafi et al. (2009) reported that methanol, n-Hexane and chloroform extracts of N. sativa effectively killed HeLa (human epithelial cervical cancer) cells by inducing apoptosis. Effenberger et al. (2010) tested terpene-terminated 6-alkyl residues of TQ on multidrug-resistant KB-V1/Vb1 cervical carcinoma and found the derivatives inducing cell death by apoptosis.
Molecular mechanisms of N. sativa action against cancer
Cancers are abnormal cell growth caused by genetic alteration. So, any agent which has anti-cancer activity, either protect genetic material from alteration or kill the genetically altered cancer cells. The active ingredients (mainly TQ) from N. sativa act on cancer cell to help to kill them by several molecular pathways (Figure 2).
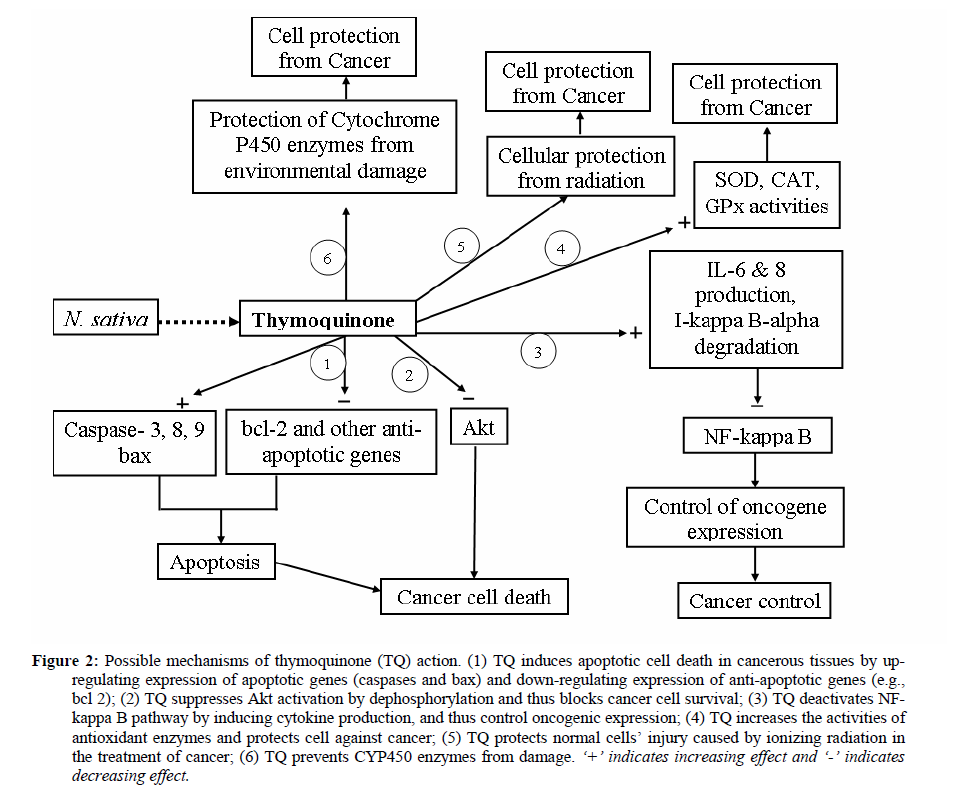
El-Mahdy et al. (2005) suggested the apoptotic mechanisms behind the anti-proliferative effect of TQ (from N. sativa) on myeloblastic leukemia HL-60 cells. They reported that TQ induces apoptosis, disrupts mitochondrial membrane potential and triggers the activation of caspases 8, 9 and 3 in HL-60 cells. The apoptosis induced by TQ was inhibited by a general caspase inhibitor, z-VAD-FMK; a caspase-3-specific inhibitor, z-DEVD-FMK; as well as a caspase-8-specific inhibitor, z-IETD-FMK. Moreover, the caspase-8 inhibitor blocked the TQ-induced activation of caspase-3, PARP cleavage and the release of cytochrome c from mitochondria into the cytoplasm. In addition, TQ treatment of HL-60 cells caused a marked increase in Bax/Bcl2 ratios due to upregulation of Bax and downregulation of Bcl2 proteins. Their results indicated that TQ-induced apoptosis is associated with the activation of caspases 8, 9 and 3, with caspase-8 acting as an upstream activator and activated caspase-8 initiates the release of cytochrome c during TQ-induced apoptosis. TQ action was also found as pro-apoptotic against colon cancer cell line HCT116 (Gali-Muhtasib et al., 2004). It was showed that the apoptotic effects of TQ are modulated by Bcl-2 protein and are linked to and dependent on p53. TQ also down-regulates the expression of NF-kappa B-regulated antiapoptotic (IAP1, IAP2, XIAP Bcl-2, Bcl-xL, and survivin) gene products (Sethi et al., 2008). Torres et al. (2010) found TQ inducing apoptosis by the activation of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase pathways in pancreatic cancer cell.
TQ has also been reported to be active in controlling Akt pathway. Yi et al. (2008) found that TQ effectively inhibited human umbilical vein endothelial cell migration, invasion, and tube formation by suppressing the activation of AKT and extracellular signal-regulated kinase. Xuan et al. (2010) found that LPS (lipopolysaccharides: a bacterial component)-induced phosphorylation of prosurvival kinases Akt and ERK1/2 was abrogated by TQ in dendritic cells.
NF-kappa B plays a key role in regulating the immune response, and incorrect regulation of NF-kappa B has been found to be linked to cancer (Albensi and Mattson, 2000). Sethi et al. (2008) found that TQ suppressed tumor necrosis factor-induced NF-kappa B activation in a dose- and time-dependent manner and inhibited NF-kappa B activation induced by various carcinogens and inflammatory stimuli. The suppression of NF-kappa B activation is correlated with sequential inhibition of the activation of I kappa B alpha kinase, I kappa B alpha phosphorylation, I-kappa-B-alpha degradation, p65 phosphorylation, p65 nuclear translocation, and the NF-kappa B-dependent reporter gene expression. Also Oberg et al. (2009) reported that a herbal melanin (HM) from N. sativa modulates cytokine production and suggested it as a ligand for TLR4 (toll-like receptor 4). They investigated the possibility that the HM-induced cytokine production is via an NF-kappa B signaling pathway and found that HM induced the degradation of I kappa B-alpha, a key step in the activation of NF-kappa B. Moreover, addition of I kappa B kinase (IKK) specific inhibitors effectively inhibited the observed HM-induced production of IL-8 and IL-6 by TLR4-transfected HEK293 (embryonic kidney 293) cells and THP-1 (Human acute monocytic leukemia) cells (Oberg et al., 2009).
Many studies showed that N. sativa oil or TQ has antioxidant activity and increases the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) glutathione peroxidase (GPx) etc (Ebru et al., 2008; Barron et al., 2008; Ismail et al., 2010). And antioxidant enzymes are clearly related to cancer- mostly their increased activities are beneficial against different types of cancer (Khan et al., 2010). Administration of N. sativa oil or TQ can lower the toxicity of other anti- cancer drugs (for example, cyclophosphamide) by an up-regulation of antioxidant mechanisms, indicating a potential clinical application for these agents to minimize the toxic effects of treatment with anticancer drugs (Alenzi et al., 2010).
In addition to these cancer inhibiting properties, components of N. sativa have cancer protective roles. Ibrahim et al. (2008) reported that N. sativa oil administration has a protective effect against the CCl4-mediated suppression of CYP (drug- metabolizing cytochrome P450 enzymes). And genetic abnormalities and polymorphisms of CYP enzymes are associated with cancer (Sim and Ingelman-Sundberg, 2006; Chen et al., 2008). Radiotherapy is one of the most common strategies for treating human cancers but this treatment is somehow risky for normal tissue. Cemek et al. (2006) showed that N. sativa and glutathione treatment significantly antagonize the effects of radiation. Therefore, N. sativa may be a beneficial agent in protection against ionizing radiation-related tissue injury. Assayed (2010) investigated the radio-protective potential of N. sativa crude oil against hemopoietic adverse effects of gamma irradiation. He found that irradiation resulted in significant reduction in hemolysin antibodies titers and delayed type hypersensitivity reaction of irradiated rats, in addition to significant leukopenia and significant decrease in plasma total protein and globulin concentration and depletion of lymphoid follicles of spleen and thymus gland. Furthermore, there was a significant increase in malondialdehyde concentration with a significant decrease in plasma GPx, CAT and erythrocyte SOD activities. But oral administration of N. sativa oil before irradiation considerably normalized all the above- mentioned criteria; and produced significant regeneration in spleen and thymus lymphoid follicles. Thus N. sativa oil is recognized as a promising natural radioprotective agent against immunosuppressive and oxidative effects of ionizing radiation.
Concluding remarks
The anti-cancer activities of N. sativa components were recognized thousands of years ago but proper scientific research with this important traditional medicine is a very recent story. More research works should be emphasized behind this because it is a safe and promising anticancer agent. Especially, researchers should investigate the active ingredients more broadly, because, there are very few authentic reports about the chemical composition of seeds or oil of N. sativa exist. Also, the exact molecular mechanisms of thymoquinone and other components on different cancers should be investigated with more emphasis because current understandings are mostly unclear. For example, it is reported that N. sativa oil can protect cells from radiation, but the molecular mechanisms behind this is not properly understood. Currently, in some parts of the world, there is a renaissance of interest in traditional remedies. Many investigators now believe that traditional medicine is a promising source of new therapeutics against cancer. Extensive research with N. sativa may contribute to the discovery of new anticancer strategies.
Reference:
Khan, Md Asaduzzaman et al. “Anticancer activities of Nigella sativa (black cumin).” African journal of traditional, complementary, and alternative medicines : AJTCAM vol. 8,5 Suppl (2011): 226-32. doi:10.4314/ajtcam.v8i5S.10
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3252704/

