Author: Abdullah O. Bamosa Department of Physiology, College of Medicine, University of Dammam, Dammam, Kingdom of Saudi Arabia
Correspondence: Dr. Abdullah O. Bamosa, University of Dammam, P.O. Box 2114, Dammam 31451, Kingdom of Saudi Arabia.
E-mail: bamosa@uod.edu.sa
ABSTRACT
Nigella sativa (black seeds) is a very famous and popular herb used for centuries in many communities. N. sativa has been shown to possess therapeutic potential to many illnesses. Hypoglycemic effect of N. sativa has been studied extensively in the literature. This review gathered and analyzed the results reported in the literature related to the hypoglycemic effect of N. sativa.
A search was done for N. sativa and black seeds as key words in PubMed and Google scholar databases. Published studies document a hypoglycemic effect of N. sativa in normal and diabetic animals and humans. Volatile oil and thymoquinone seem to be the most effective fractions of the seed in producing its hypoglycemic effect. The mechanism of . sativa hypoglycemic effect is multifactorial including increasing insulin level, decreasing insulin resistance, stimulating β cells activity, direct insulin-like effect, and decreasing intestinal glucose absorption. Further, basic followed by clinical research to explore N. sativa ingredient responsible for its promising hypoglycemic effect is recommended.
Key words: Black seeds, diabetes mellitus, hypoglycemic effect, Nigella sativa, thymoquinone, volatile oil
![]() INTRODUCTION
INTRODUCTION
In recent years, there has been significant progress and development in diagnosing and treating many illnesses using the techniques and standard drugs in modern medicine. However, there are many illnesses to which modern medicine could not find treatment. Furthermore, most drugs have unwanted or very serious side effects. In view of such a situation, people started to look for treatments in traditional and herbal medicine. Nigella sativa is one of the famous medicinal plants that have been used for treatment of various illnesses in different parts of the world.[1]N. sativa (black seeds) is a seasonal plant, belonging to the Ranunculaceae family, mostly grown in the Middle East, Africa and Indian Subcontinent.[2] N. sativa research is growing over the years and the number of publications related to the beneficial effects of N. sativa in health and disease is now exceeding 500 publications. Several reviews have been published recently in relation to the beneficiary effects of N. sativa in different conditions such as anticancer,[3] anti-inflammatory,[4] cardiovascular,[5] renal,[6] immunomodulation,[7] antidiabetic[8] as well as many other general reviews.[1,2,9,10] This review is addressing the hypoglycemic effect of N. sativa and its active ingredient thymoquinone, an area, which has not been covered comprehensively in the literature to-date.
COMPOSITION AND TOXICITY
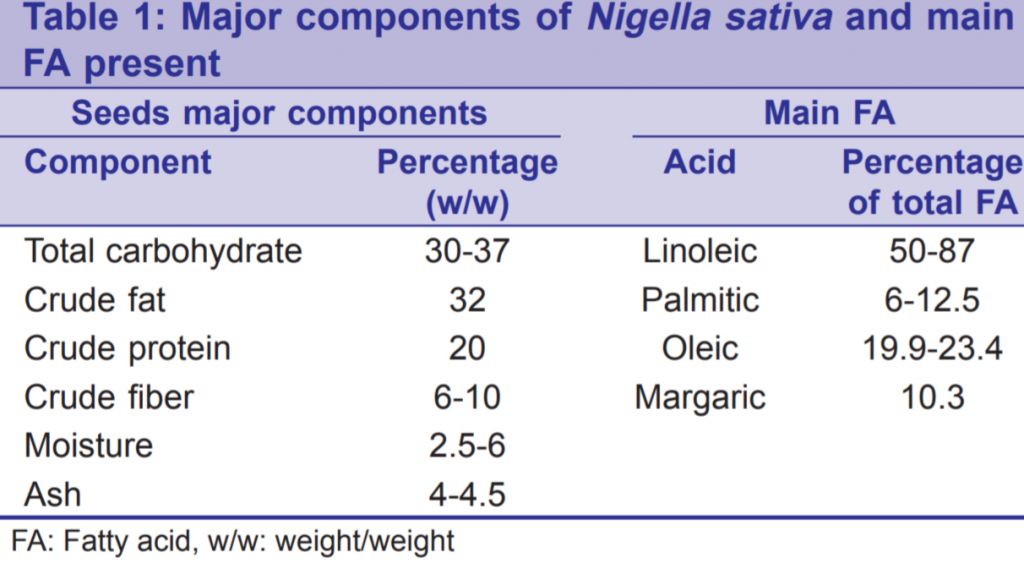
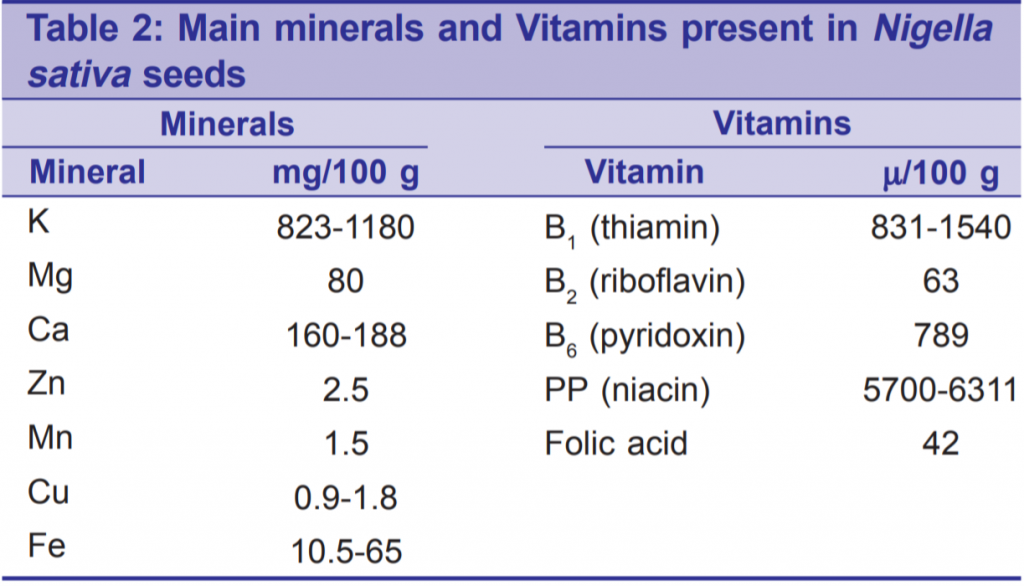
Nigella sativa is rich with many important nutrients, vitamins, minerals, and chemical compounds of potential therapeutic effects. Table 1 shows the major components of N. sativa with the main fatty acids (FAs) constituents.[11-15] Linoleic acid, unsaturated FA, being the most abundant gives some explanation for the certain therapeutic and nutritive values of the seeds. Table 2 shows the analysis of minerals and vitamins in these valuable seeds.[11,12,16] Such a composition would explain the antioxidant power of the seeds. The aromatic smell and the special flavor of the seed are attributed to its volatile oil. The seeds contain fixed oil (36-38%), alkaloids, saponins, and essential (volatile) oil (0.4-2.5%).[1] The major ingredients of essential oil are: Thymoquinone (27.8- 57%), p-cymene (7.1-15.5%) and carvacrol (5.8-11.6%).[1] Thymoquinone has been analyzed chemically and is already manufactured for research purposes.
Toxicological studies for N. sativa and thymoquinone revealed extremely low toxicity. LD50 values for a single oral and intraperitoneal (IP) dose of N. sativa oil in mice were 28.8 mL/kg and 2.06 mL/kg, respectively.[17] LD50 for acute oral administration of thymoquinone in mice was 0.87 g/kg[18] and 2.4 g/kg.[19] A figure of 0.794 was obtained for LD50 values in rat which were close to those in mice.[18] Subacute oral administration of 90 mg/kg day of thymoquinone in mice for 90 days revealed no toxicity.[19] IP LD50 for thymoquinone was 104.7 mg/kg for mice and
57.5 mg/kg for rat.[18] These studies showed a wide margin of safety for N. sativa and its active ingredient thymoquinone.
EFFECT OF NIGELLA SATIVA AND THYMOQUINONE ON BLOOD GLUCOSE IN NORMAL ANIMALS
Powdered N. sativa in a dose of 36 mg/200 g rat did not produce any significant effect on normal rat’s blood glucose level.[20] N. sativa extract, equivalent to a dose of 40 mg/day, did not produce any significant effect on blood glucose in normal rats.[21] In agreement with these negative results, petroleum ether extract of N. sativa produced no significant effect on fasting plasma glucose.[22] However, the authors reported a significant reduction in fasting plasma insulin which they attributed to sensitizing liver cells to insulin.[22] On the other hand, other researchers reported conflicting results. Al-Hader et al. found a significant hypoglycemic effect in normal rabbits produced by daily 50 mg/kg IP injection of volatile oil.[23] Authors reported that the effect was only shown on the morning of the seventh day and was time- dependent. However, since authors did not measure blood glucose daily, this conclusion is questionable. The hypoglycemic effect observed in this study could have been transient and faded out over the previous night. Hence, the authors found no hypoglycemic effect at 0 hour measurement in the morning of the 7th day.[23] The same hypoglycemic effect was reported in normal rats treated with 1 mL/kg body weight of N. sativa fixed oil for 12 weeks.[24] Our group found a strong hypoglycemic effect produced by N. sativa and thymoquinone in normal rats.[25] Six doses of N. sativa and five doses of thymoquinone were used for five durations (1, 4, 7, 1 and 14 days). The authors reported no effect in most durations by 50 mg/day N. sativa and 0.5 mg/ day thymoquinone.[25] However, 100-300 mg/day N. sativa and thymoquinone 1-6 mg/day produced highly significant glucose lowering effects which started on the 4th day for both N. sativa and thymoquinone.[25] These conflicting results in normal animals are most probably due to different doses used in the reported studies. It seems that the glucose-lowering effect of N. sativa seeds starts from a dose not <50 mg/200 g rat/day. Hence, majority of researchers reporting negative results in normal animals were using sub-therapeutic doses of N. sativa.
EFFECT OF NIGELLA SATIVA
AND THYMOQUINONE ON BLOOD GLUCOSE IN DIABETIC ANIMALS
Majority of studies on the hypoglycemic effect of N. sativa and thymoquinone in diabetic animals showed positive results. However, two studies reported no significant effect of N. sativa on blood glucose in alloxan[20] and in streptozotocin (STZ) induced diabetic rats.[21] Both groups were using low dose of N. sativa (below 50 mg/200 g rat/day), which could explain their negative results.
A plant mixture, which contained N. sativa, produced a strong hypoglycemic effect in STZ diabetic rats.[26] The authors attributed this hypoglycemic effect of the plant mixture to a decrease in glucogenesis. Another group of researchers using another plant mixture, which contained N. sativa, reported a significant glucose lowering effect.[27]
Intragastric N. sativa oil administration to nicotinamide- induced diabetic hamster in a dose of 400 mg/kg/day revealed a significant decrease in blood glucose and elevation in insulin.[28] The same group of researchers using the same dose of oil and animal model, reported a time-dependent hypoglycemic effect (1-4 weeks) with a decrease in hepatic gluconeogenesis.[29] Oral administration of 500 mg/kg N. sativa oil to STZ diabetic rats decreased fasting blood glucose significantly with insignificant increase in insulin.[30] IP injection of 0.2 mL/kg of N. sativa oil for 30 days in STZ diabetic rats, produced a significant reduction in fasting blood glucose associated with enhanced antioxidant power.[31] Collectively, the aforementioned studies showed a significant hypoglycemic effect produced by N. sativa fixed oil in diabetic animal models in an oral dose of 400-500 mg/kg/day and IP dose of 0.2 mL/kg.
Nigella sativa aquous extract (20 mL/kg) decreased blood glucose from 340 ± 45.26 to 194.41 ± 27.33, while decreasing malondialdehyde and elevating gluthathione in alloxan-induced diabetic rabbits.[32] IP administration of 5 mg/kg hydroalcholic (1-32 days) extract of N. sativa produced a time-dependent hypoglycemic effect in STZ diabetic rats associated with protection of b cells.[33] Ethanol extract of N. sativa in a dose of 300 mg/kg (orally) produced a significant hypoglycemic effect and improved the antioxidant status of STZ diabetic rats.[34] Aqueous extract of N. sativa (2 mL/kg IP) induced significant hypoglycemic effect on the 10th day associated with improved antioxidant status and preservation of islet structure in STZ diabetic rats.[31]
Nigella sativa volatile oil was the most frequently used component of N. sativa in investigating its hypoglycemic effect in diabetic animal models. IP injection of 50 mg/kg of N. sativa volatile oil to alloxan-diabetic rats induced a time-dependent hypoglycemic effect shown at 4 and 6 hour measurements, which was associated with no change in insulin.[23] The authors stated that the effect of the N. sativa volatile oil took 6 days to appear. However, this conclusion may not be accurate as the authors did not measure blood sugar before the 7th day.[23] The hypoglycemic effect of N. sativa oil could have been transient and did not last to the next morning, which led to no significant difference in blood sugar level at 0 hour in the morning of the 7th day.[23] IP injection of N. sativa volatile oil (10 mg/kg/day) to STZ diabetic rats produced time-dependent reduction in blood glucose with parallel increase in number of insulin-immunoreactive b cells and their granules over the 30 days duration.[35] Another group of investigators, utilizing same experimental protocol, reported hypoglycemic effect of N. sativa volatile oil associated with amelioration of oxidative stress and the preservation of pancreatic b cells integrity.[36] The same dose of N. sativa volatile oil (10 mg/kg) administered orally to STZ diabetic rats protected pancreatic b cells against STZ-induced damage.[37] IP injection of 100 mg/kg of N. sativa volatile oil for 30 days to cadmium- treated rats induced significant hypoglycemic effect with a parallel increase in insulin and b cell integrity.[38,39]
Thymoquinone was intragastrically-administered to STZ nicotinamide-induced diabetic rats at 20, 40, 80 mg/kg by week for 45 days showed significant, dose dependent, hypoglycemic effect with a decrease in HbA1c and elevation in insulin.[40] Interestingly, blood glucose was almost normalized by 80 mg/kg thymoquinone and this dose of thymoquinone could also normalize the glucose tolerance test of these diabetic rats.[40]
EFFECT OF NIGELLA SATIVA ON BLOOD GLUCOSE IN HUMANS
The first report on the effect of N. sativa on blood glucose level in humans found in the literature was in 1997 by Bamosa et al.[41] The authors used 2 g/day of powdered N. sativa capsules for 2 weeks on volunteer university students. They reported a transient significant decrease in blood glucose level by the end of the 1st week, which then went up to be insignificantly below baseline by the end of the 2nd week.[41] Another group of investigators reported a significant hypoglycemic effect produced by 2.5 ml of N. sativa oil given twice daily for 6 weeks in patients with metabolic syndrome.[42] The same group of researchers reported a significant improvement in glycemic control induced by 8 weeks treatment with 500 mg/day of powder N. sativa capsules, as an adjuvant in patients with metabolic syndrome associated with type II diabetes mellitus.[43] Bamosa et al., in a study of 3 months treatment with 3 doses of powdered N. sativa (1, 2 and 3 g/day) in type II diabetic patients, reported significant reduction in HbA1c, fasting and random blood glucose levels induced by 2 and 3 g/ day doses.[44] However, 1 g/day dose failed to induce a significant improvement in glycemic control in these type II diabetic patients, conflicting with the previous study of Najmi et al. Two differences between the two studies could account for this discrepancy; sample size was more in Najmi et al. (40 patients) than Bamosa group (25 patients). Furthermore, the patients’ condition differ significantly (metabolic syndrome vs. type II diabetes). It could be that metabolic syndrome patients with type II diabetes mellitus are benefiting more from lower doses of N. sativa than the pure type II diabetes mellitus patients.
Supplementation of powdered N. sativa to adults failed to show significant decrease in blood glucose which was attributed to small sample size.[45] However, this study lacks inclusion/exclusion criteria and therefore, sample size could have been adequate but lacking uniformity in confounding factors. Bamosa group in a recent study conducted on uncontrolled type II diabetic patients, administered 2 g/day of powdered N. sativa as adjuvant to oral hypoglycemic drugs for 1-year, found a significant improvement in glycemic control observed by a decrease in HbA1c and both fasting and random blood glucose. Interestingly, in both studies conducted by Bamosa group (3 months and 1 year) the effect on fasting blood glucose was seen after 1 day of N. sativa administration in those diabetic patients. This shows that the effect of N. sativa on blood glucose is fast in humans and it could be so in animals.
MECHANISM OF NIGELLA SATIVA HYPOGLYCEMIC EFFECT
Balance concept for any variable in the body is based on equalization of its input to its output. Glucose plasma input could be through intestinal absorption, endogenous production (glycogenolysis and gluconeogenesis), while output from plasma is contributed by tissues glucose utilization (glycolysis, pentose phosphate pathway, tricarboxylic acid cycle and glycogen synthesis).[41,46] Several hormones, insulin being the most important one, play an important role in glucose hemostasis. The normal function of insulin releasing cells (b cells in pancreas), as well as other cellular mechanisms are distorted by oxidative stress which would contribute in induction of diabetes mellitus and its complications.[47-49] Several studies have reported a powerful antioxidant effect of Nigella sativa and its constituents, which they proposed to contribute to its hypoglycemic effect.[30-32,34,36] The enhancement of antioxidant power in diabetics might lead to preservation of islet cells and cellular mechanism involved in glucose hemostasis. Indeed, numerous histopathological studies on different species with different diabetic models, have reported preservation of b cells in animals treated with N. sativa and its derivatives.[28,31-33,35-39] As expected in all of the aforementioned studies this preservation of islet cells was associated with significant increase in insulin. However, in conflict with these studies, there were no significant changes in basal insulin level in alloxan- diabetic rabbits treated with N. sativa volatile oil[23] and in STZ diabetic rats treated with N. sativa oil, nigellone or thymoquinone.[50]
There is no clear explanation for the discrepancy between these two studies with the previous nine studies reporting insulin elevation. However, isolated rat pancreatic islet cells produced dose-dependent elevation in insulin in the presence of 8.3 mmol/L glucose by different fractions of N. sativa.[51]
Hepatic glucose production from gluconeogenic precursors (alanine, glycerol and lactate) was significantly lower in STZ-induced diabetic hamster treated with N. stiva oil.[29] Another group of researchers attributed the hypoglycemic effect induced by N. sativa oil in alloxan- induced diabetic rats to deceased gluconeogenesis.[52] Going in the same line of decreased glucose input, Meddah et al. reported direct inhibition of electrogenic intestinal absorption of glucose in vitro by N. sativa extract.[53] Thymoquinone decreased gluconeogenic liver enzymes activities while elevating glycolytic and lipogenic enzymes in STZ nicotinamide-induced diabetic rats.[40]
Nigella sativa ethanol extract was reported to induce an important insulin-like stimulation of glucose uptake in cultured cell lines of skeletal muscle cells and adipocytes following an 18 hour treatment.[54] The same researchers working on three cell lines; skeletal muscles, hepatocytes and adipocytes, proposed multiple molecular targets to account for this insulin-like effect.[55] Neuron-specific enolase (NSE), in the absence of insulin, increased basal phosphorylation of AKt, a key mediator of the effects of insulin, in skeletal muscle cells and liver cells.[55] They also found activation of AMP-activated protein kinase, a metabolic enzyme with insulin-like effects, induced by NSE in both skeletal muscle cells and hepatocytes but not in adipocytes.[55] NSE behaved like an agonist of PPART in adipocytes which could account, at least in part, for adipogenesis produced by norepinephrine.[55]
It could be deduced from these studies that the mechanism of N. sativa hypoglycemic effect is multifactorial including: Amelioration of oxidative stress, elevation of insulin, attenuation of insulin resistance and hepatic gluconeogenesis and direct insulin-like effects at the cellular and molecular levels in various organs. Results of the studies presented in this review showed a promising hypoglycemic effect of N. sativa and many of its derivatives. N. sativa volatile oil and thymoquinone might be the most potent fractions of N. sativa in producing its hypoglycemic effect. Further toxicological and molecular studies on various fractions of the seeds are needed to find out the safe and effective ingredient of N. sativa to be used in future clinical trials on diabetic patients
REFERENCES
- Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2003;17:299-305.
- Khan MA. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 1999;7:15-35.
- Khan MA, Chen HC, Tania M, Zhang DZ. Anticancer activities of Nigella sativa (black cumin). Afr J Tradit Complement Altern Med 2011;8:226-32.
- Woo CC, Kumar AP, Sethi G, Tan KH. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem Pharmacol 2012;83:443-51.
- Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc Toxicol 2013;13:9-21.
- Ragheb A, Attia A, Eldin WS, Elbarbry F, Gazarin S, Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti- inflammatory agent, against renal injury: A review. Saudi J Kidney Dis Transpl 2009;20:741-52.
- Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol 2005;5:1749-70.
- Mathur ML, Gaur J, Sharma R, Haldiya KR. Antidiabetic properties spice plant Nigella sativa. Journal of Endocrinology and Metabolism 2011;1:1-8.
- Randhawa MA, Al-Ghamdi MS. A review of the pharmaco- therapeutic effects of Nigella sativa. Pak J Med Res 2002;41:77-83.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed 2013;3:337-52.
- Al-Jasass FM, Al-Jasser MS. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. ScientificWorldJournal 2012;2012:859892.
- Nergiz C, Otles S. Chemical composition of Nigella sativa L. seeds. Food Chem 1993;48:259-61.
- Liu X, Abd El-Aty AM, Cho SK, Yang A, Park JH, Shim JH. Characterization of secondary volatile profiles in Nigella sativa seeds from two different origins using accelerated solvent extraction and gas chromatography-mass spectrometry. Biomed Chromatogr 2012;26:1157-62.
- Amin S, Mir SR, Kohli K, Ali B, Ali M. A study of the chemical composition of black cumin oil and its effect on penetration enhancement from transdermal formulations. Nat Prod Res 2010;24:1151-7
- Nickavar B, Mojab F, Javidnia K, Amoli MA. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z Naturforsch C 2003;58:629-31.
- Ghosheh OA, Houdi AA, Crooks PA. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J Pharm Biomed Anal 1999;19:757-62.
- Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002;9:69-74.
- Al-Ali A, Alkhawajah AA, Randhawa MA, Shaikh NA. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad 2008;20:25-7.
- Badary OA, Al-Shabanah OA, Nagi MN, Al-Bekairi AM, Elmazar MM. Acute and subchronic toxicity of thymoquinone in mice. Drug Dev Res 1998;44:56-61.
- Abdel-Rahman ME, Abd El-Raouf ME. A study of some biological activities of Nigella sativa (black seeds): Habat El Baraka. J Egypt Soc Pharmacol Exp Ther 1992;11:781-800.
- Al-Awadi FM, Gumaa KA. Studies on the activity of individual plants of an antidiabetic plant mixture. Acta Diabetol Lat 1987;24:37-41.
- Le PM, Benhaddou-Andaloussi A, Elimadi A, Settaf A, Cherrah Y, Haddad PS. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J Ethnopharmacol 2004;94:251-9.
- Al-Hader A, Aqel M, Hasan Z. Hypoglycemic effects of the volatile oil of Nigella sativa seeds. Int J Pharmacogn 1993;31:96-100.
- Zaoui A, Cherrah Y, Alaoui K, Mahassine N, Amarouch H, Hassar M. Effects of Nigella sativa fixed oil on blood homeostasis in rat. J Ethnopharmacol 2002;79:23-6.
- Hawsawi ZA, Ali BA, Bamosa AO. Effect of Nigella sativa (black seed) and thymoquinone on blood glucose in albino rats. Ann Saudi Med 2001;21:242-4.
- al-Awadi F, Fatania H, Shamte U. The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res 1991;18:163-8.
- Eskander EF, Jun HW, Ibrahim KA, Abdelal WE. Hypoglycemic effect of a herbal formulation in alloxan induced diabetic rats Egypt. J Pharm Sci 1995;36:253-70.
- Fararh KM, Atoji Y, Shimizu Y, Takewaki T. Isulinotropic properties of Nigella sativa oil in streptozotocin plus nicotinamide diabetic hamster. Res Vet Sci 2002;73:279-82.
- Fararh KM, Atoji Y, Shimizu Y, Shiina T, Nikami H, Takewaki T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res Vet Sci 2004;77:123-9.
- Salama RH. Hypoglycemic effect of lipoic acid, carnitine and Nigella sativa in diabetic rat model. Int J Health Sci (Qassim) 2011;5:126-34.
- Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic ß-cells of streptozotocin-induced diabetic rats. J Diabetes 2010;2:256-66.
- Meral I, Yener Z, Kahraman T, Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti-oxidant defence system and liver damage in experimentally-induced diabetic rabbits. J Vet Med A Physiol Pathol Clin Med 2001;48:593-9.
- Alimohammadi S, Hobbenaghi R, Javanbakht J, Kheradmand D, Mortezaee R, Tavakoli M, et al. Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: An experimental study with histopathological evaluation. Diagn Pathol 2013;8:137.
- Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J Exp Biol 2006;44:745-8.
- Kanter M, Meral I, Yener Z, Ozbek H, Demir H. Partial regeneration/proliferation of the beta-cells in the islets of Langerhans by Nigella sativa L. in streptozotocin-induced diabetic rats. Tohoku J Exp Med 2003;201:213-9.
- Kanter M, Coskun O, Korkmaz A, Oter S. Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol 2004;279:685-91.
- Kanter M, Akpolat M, Aktas C. Protective effects of the volatile oil of Nigella sativa seeds on beta-cell damage in streptozotocin- induced diabetic rats: A light and electron microscopic study. J Mol Histol 2009;40:379-85.
- Demir H, Kanter M, Coskun O, Uz YH, Koc A, Yildiz A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium-treated rats. Biol Trace Elem Res 2006;110:151-62.
- Altan MF, Kanter M, Donmez S, Kartal ME, Buyukbas S. Combination therapy of Nigella sativa and human parathyroid hormone on bone mass, biomechanical behavior and structure in streptozotocin-induced diabetic rats. Acta Histochem 2007;109:304-14.
Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sciences 2009;85:830-4.
- Bamosa AO, Ali BA, Sowayan SA. Effect of oral ingestion of Nigella sativa seeds on some blood parameters. Saudi Pharm J 1997;5:2-3.
- Najmi A, Nasiruddin M, Khan RA, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries 2008;28:11-4.
- Najmi A, Nasiruddin M, Khan RA, Haque SF. Therapeutic effect of Nigella sativa in patients of poor glycemic control. Asian J Pharm Clin Res 2012;5:224-8.
- Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol 2010;54:344-54.
- Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: Results of a randomized, double-blind controlled trial. J Altern Complement Med 2009;15:639-44.
- Shirwaikar A, Rajendran K, Barik R. Effect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitus. J Ethnopharmacol 2006;107:285-90.
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40:405-12.
- Oberley LW. Free radicals and diabetes. Free Radic Biol Med 1988;5:113-24.
- Szaleczky E, Prechl J, Fehér J, Somogyi A. Alterations in enzymatic antioxidant defence in diabetes mellitus — A rational approach. Postgrad Med J 1999;75:13-7.
- El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of Nigella sativa oil is mediated by extrapancreatic actions. Planta Med 2002;68:465-6.
- Rchid H, Chevassus H, Nmila R, Guiral C, Petit P, Chokaïri M, et al. Nigella sativa seed extracts enhance glucose-induced insulin release from rat-isolated Langerhans islets. Fundam Clin Pharmacol 2004;18:525-9.
- Houcher Z, Boudiaf K, Benboubetra M, Houcher B. Effects of methanolic extract and commercial oil of Nigella sativa l. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines 2007;18:8-18.
- Meddah B, Ducroc R, El Abbes Faouzi M, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol 2009;121:419-24.
- Benhaddou-Andaloussi A, Martineau LC, Spoor D, Vuong T, Leduc C, Joly E, et al. Antidiabetic activity of Nigella sativa seed extract in cultured pancreatic cells, skeletal muscle, and adipocytes. Pharm Biol 2008;46:96-104.
- Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, et al. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte, and liver cells. Diabetes Obes Metab 2010;12:148-57

